(gas laws: Gay-Lussac, Amontons, Boyle)
Item no.: P2320160
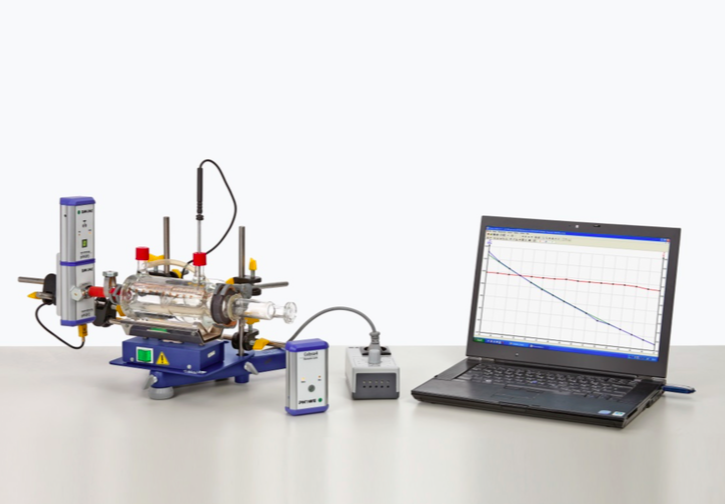
Principle
The state of a gas is determined by temperature, pressure and amount of substance. For the limiting case of ideal gases, these state variables are linked via the general equation of state. For a change of state under isochoric conditions this equation becomes Amontons’ law. In this experiment it is investigated whether Amontons’ law is valid for a constant amount of gas (air).
Tasks
– For a constant amount of gas (air) investigate the correlation of
1. Volume and pressure at constant temperature (Boyle and Mariotte’s law)
2. Volume and temperature at constant pressure (Gay-Lussac’s law)
3. Pressue and temperature at constant volume (Charles’ (Amontons’ law))
– From the relationships obtained calculate the universal gas constant as well as the coefficient of thermal expansion, the coefficient of thermal tension, and the coefficient of cubic compressibility.
What you can learn about
– Thermal tension coefficient
– General equation of state for ideal gases
– Universal gas constant
– Amontons’ law
Software included. Computer not provided.