Item no.: P3121060
Principle
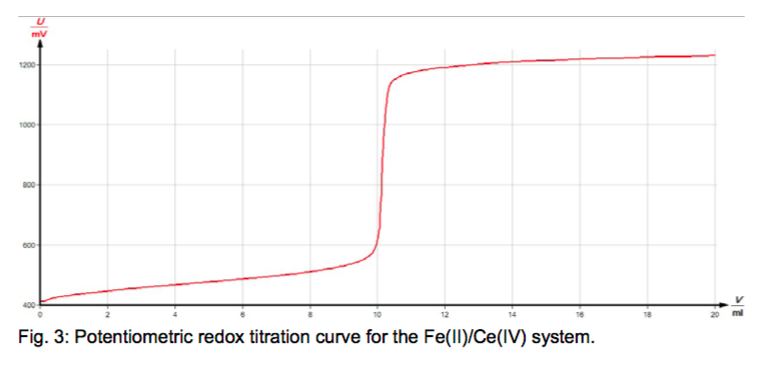
Potassium permanganate solutions which are used as oxidizing measuring solutions in redox titrations can in most cases be replaced by Ce(IV) solutions. These offer the advantages that they do not change on storage and that the course of the redox titration can be very conveniently followed by measuring the electrochemical potential. The equivalent point can then be found by determination of the inflection point of the potential curve which results from plotting the measured values.
Tasks
Titrate Iron(II) sulphate solution with Ce(IV) sulphate solution.
What you can learn about
- Redox titration
- Iron(II) sulphate
- Ce(IV) sulphate
- Titration
- Cerimetry
Necessary accessories
- Precision balance 6200g/0.01g
Software provided. Computer not included.